Why are antibodies ineffective against Omicron COVID-19?
For context, this is a question I answered on Quora
I think you mean to ask why are anti-S1 antibodies short lived and ineffective against every omicron variant? It is because they are formed against the most mutagenic part of the virus that changes every time it infects a new host and even during the course of the infection while the host receives Paxlovid treatment which itself create drug resistant strains.
Paxlovid is more like PfraudlovidPaxlovid and Remdesivir are Creating Drug Resistant COVID Variants
And every booster is not inducing antibodies solely against the new mutated spike protein, but still directing the vaccinees’ immune system to search and destroy the the ancestral variant spike protein, which has long ceased circulation, in a well documented process known as antigenic imprinting/original antigenic sin, which until 2022 was a “conspiracy theory.”
An immunological cohort analysis found that the XBB1.5 booster elicited a humoral response dominated by reactivation of pre-existing memory B cells induced by the Wuhan-Hu-1 spike, rather than production of Omicron XBB.1.5-specific new memory B cells. Just to put this in perspective, the XBB1.5 sub variant of early to mid 2023, which had vanished by the time most vaccinees got their new booster, was already several generations removed from the Wuhan-Hu-1 variant of 2019–2020.
A murine mouse model experiment found that mice with metabolic syndrome established after 12 weeks of a high fat diet and vaxxed with two 5 microgram modRNA doses not only evidenced immune imprinting towards the ancestral variant in serum sample, same as the wildtype control mice, but also diminished neutralization capacity against both the ancestral variant and delta variant compared to the wildtype control mice. Of course, this experiment only examined spike specific antibody serum levels and not the much more important cellular immune response.
A Syrian hamster model experiment found that both the XBB1.5 booster and a per-omnicron gamma variant modRNA booster created a strong but narrow neutralizing antibody response against the booster-matched variant. After the subjects were exposed to EG5.1 or JN1 sub variant they found that antibody titers increased, but remained strongly biased toward the variant used in the vaxx (evidence of antigenic imprinting).
Another rodent study published in iScience additionally finds that repeated boosters that target the receptor binding domain impairs serum neutralization, the activation of T memory cells, and leads to immune tolerance. Immune tolerance is the body’s inability to produce antigen specific antibodies that activate memory T cells or insufficient production of them upon encountering a new infection. The study detected a 2.5–4x reduction in average antibody titers against the delta and omicron variants compared to the wild type and a (statistically) significant reduction in the serum neutralization in the extended (boosted) group against all 3 types compared the the conventional (primary series) group.
Wang et al., found that there are no discernible differences in omicron neutralization between the older monovalent (used in the study above) and the new bivalent:
‘Boosting with a new bivalent mRNA vaccine targeting both BA.4/BA.5 and an ancestral SARS-CoV-2 strain did not elicit a discernibly superior virus-neutralizing antibody responses compared boosting with an original monovalent vaccine.’
After analyzing serum collected from individuals who received 3 doses of the monovalent and comparing them to those who received the bivalent (an average of 26 and 24 days after vaccination). All participants exhibited the highest neutralizing titers against the ancestral strain and there were no statistically significant differences in the neutralization of any variant between the groups 3–5 weeks after the booster.
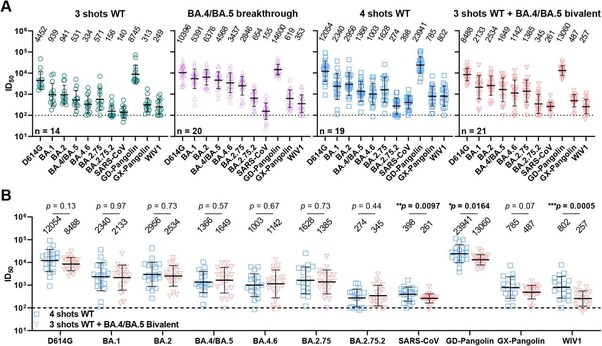
A study published in the New England Journal of Medicine (Davis-Gardner et. al.,) also analyzed serum samples from (n = 35) participants: 7–28 days after the first monovalent booster (n = 11), 6–57 days after the second monovalent booster, and 16–42 days after the bivalent booster (n = 12). The study found substantially lower neutralization activity against all omicron subvariants compared to the wildtype-ancestral variant in all three cohorts. In both monovalent cohorts (n=23)(Geometric Means) antibody titers against BA1 and BA5 were 5–9x lower than against wildtype-ancestral variant and 23-63x lower against BA.2.75.2, BQ1.1 and XBB than the wildtype-ancestral variant. In the bivalent booster cohort, (Geometric Means) antibody titers against BA1 and BA5 were 4x lower than against wildtype-ancestral variant and 12–26x lower against BA.2.75.2, BQ1.1 and XBB than the wildtype-ancestral variant.
Another study published in the New England Journal of Medicine (n = 33) found that both the monovalent (n = 15) and bivalent (n = 18) boosters lead to the expansion of antibody titers for the wildtype-ancestral variant and lower antibody titers for the BA5 omicron variant after a median of 3 doses. Memory T cell responses were barley affected in either group and the bivalent booster produced a slightly higher level of antibody titers at a rate ratio of 1.3.
Another Study published in the New England Journal of Medicine (Hachmann et al.,) confirmed that Pfizer booster produced a much more robust antibody response to wildtype-ancestral variant, with a median 5,783 antibody titers 2 weeks after administration, and a much more subdued response to the omicron subvariants with a median 900 antibody titers against BA1, median 829 antibody titers against BA2, median 410 antibody titers against BA.2.12.1, and a median 275 antibody titers against BA4 and 5 2 weeks after the booster. All but one of the participants infected with BA1 and 2 subvariants were vaccinated suggest substantial immune evasion by omicron subvariants from the booster immunization. Participants with prior infections had median antibody titers of 11,050 against wildtype-ancestral variant, 1,740 against BA1, 1,910 against BA2, 1,150 against BA.2.12.1, and 590 against BA4 and 5